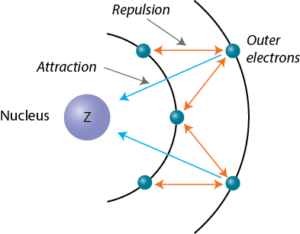
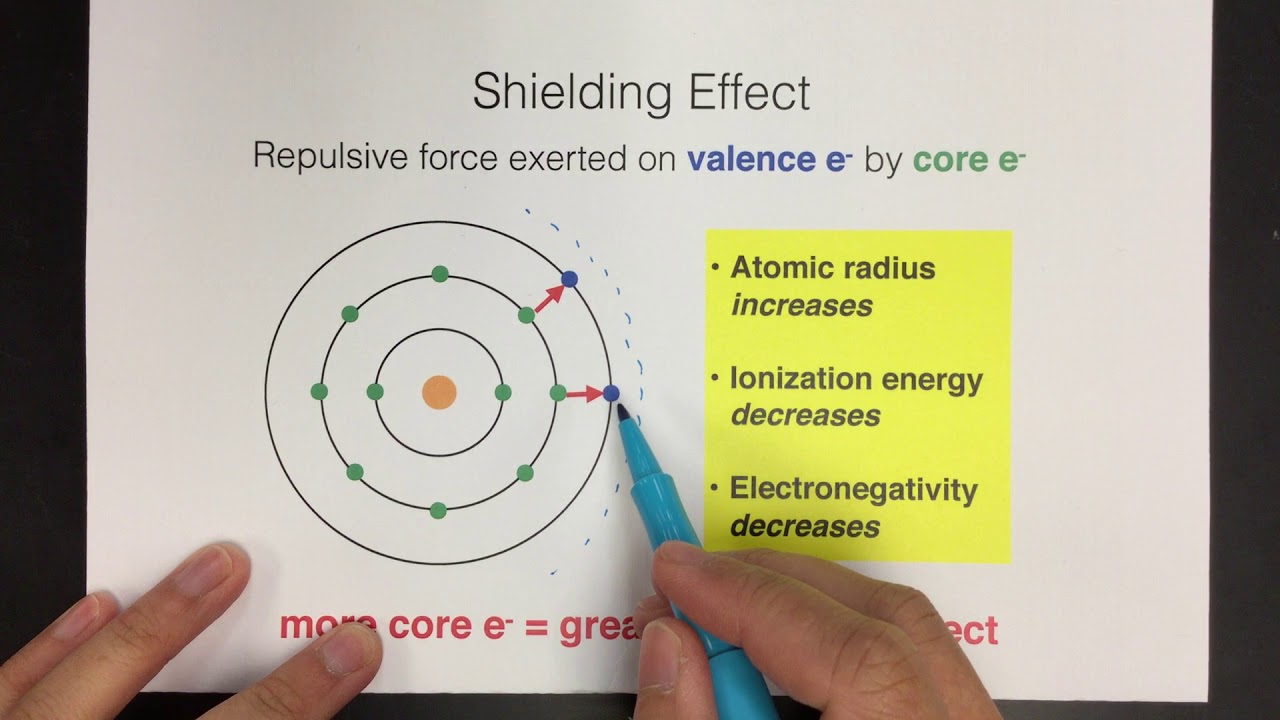
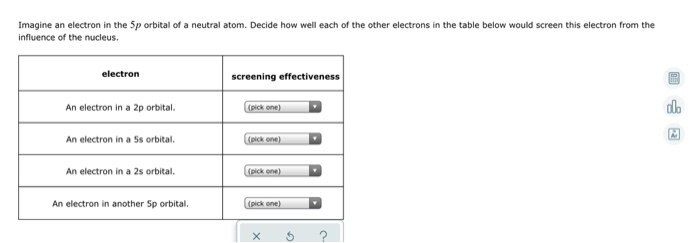
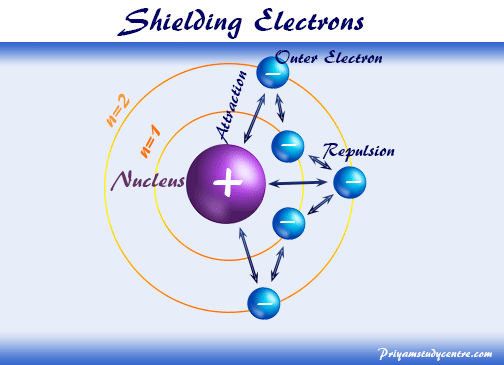
SOLVED: Question 37 (1 point) Screening of the nuclear charge by core electrons in atoms is: less efficient than by valence electrons more efficient than by valence electrons essentially identical to that

Color online) (a) Valence-electron screening cloud around the excited... | Download Scientific Diagram
Welcome to Chem Zipper.com......: Effective Nuclear charge (Z* or Zeff): Slater's rule: Screening effect or Shielding effect
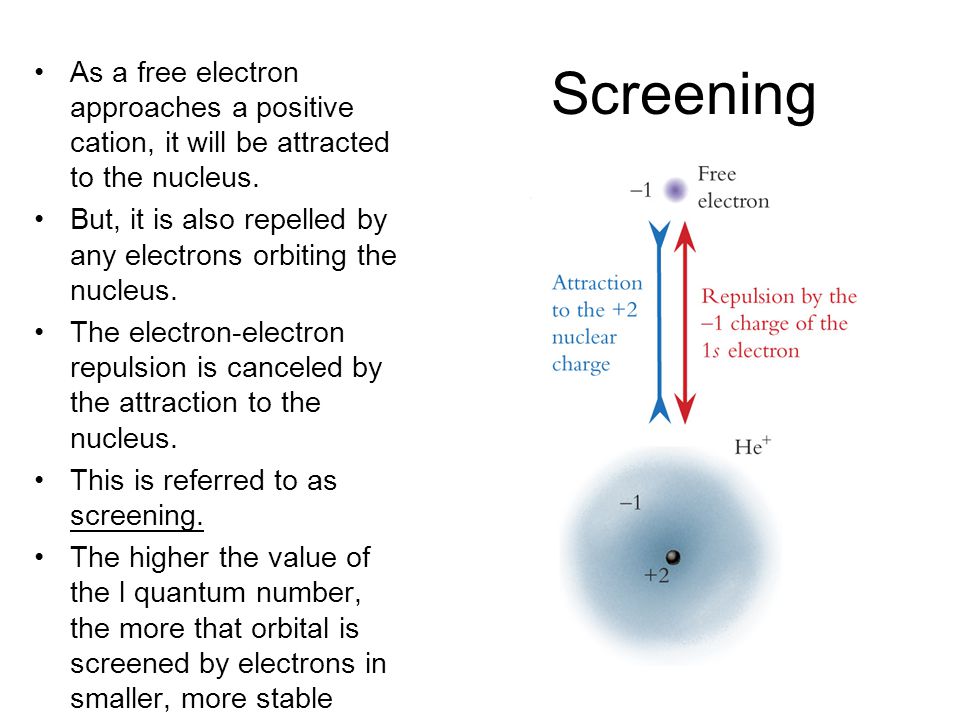
Screening As a free electron approaches a positive cation, it will be attracted to the nucleus. But, it is also repelled by any electrons orbiting the. - ppt download

What is the Difference Between Effective Nuclear Charge and Shielding Effect | Compare the Difference Between Similar Terms
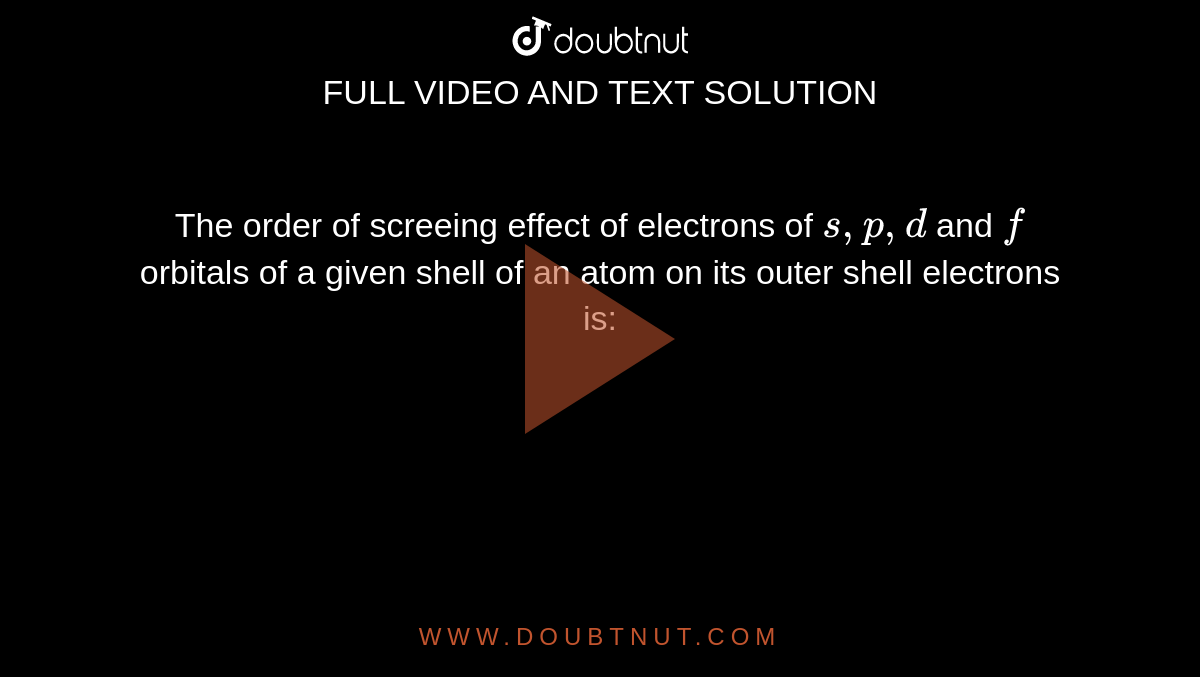
The order of screening effect of electrons of s, p, d and f orbitals of a given shell of an atom on its outer shell electrons is * Thinking process:To solve question,

SOLVED: Imagine an electron in the 5d orbital of neutral atom Decide how well each of the other electrons in the table below would screen this electron from the influence of the

![PDF] Electron screening in the liquid-gas mixed phases of nuclear matter | Semantic Scholar PDF] Electron screening in the liquid-gas mixed phases of nuclear matter | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0aca80e13ffae0a622683cab2a1f609552f53ea1/8-Figure1-1.png)

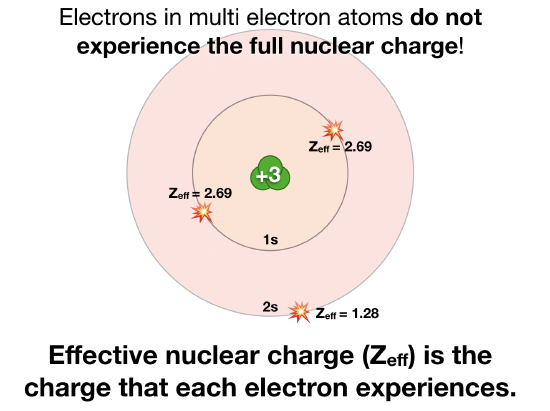
![PDF] Screening effect on electron capture in presupernova stars | Semantic Scholar PDF] Screening effect on electron capture in presupernova stars | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f3a4c8fa3e34c91ec5eb0aa97d7de16775061f23/2-Figure1-1.png)