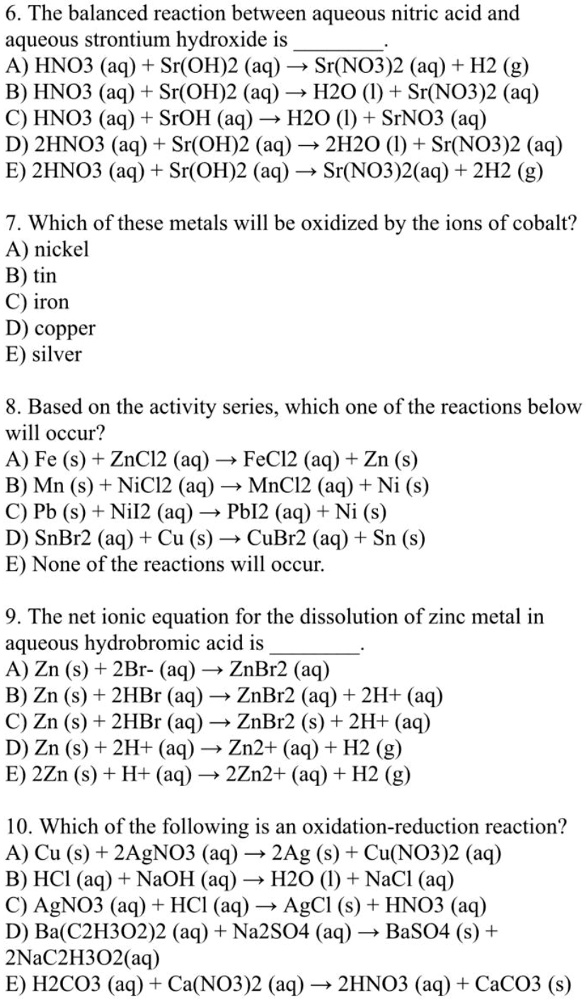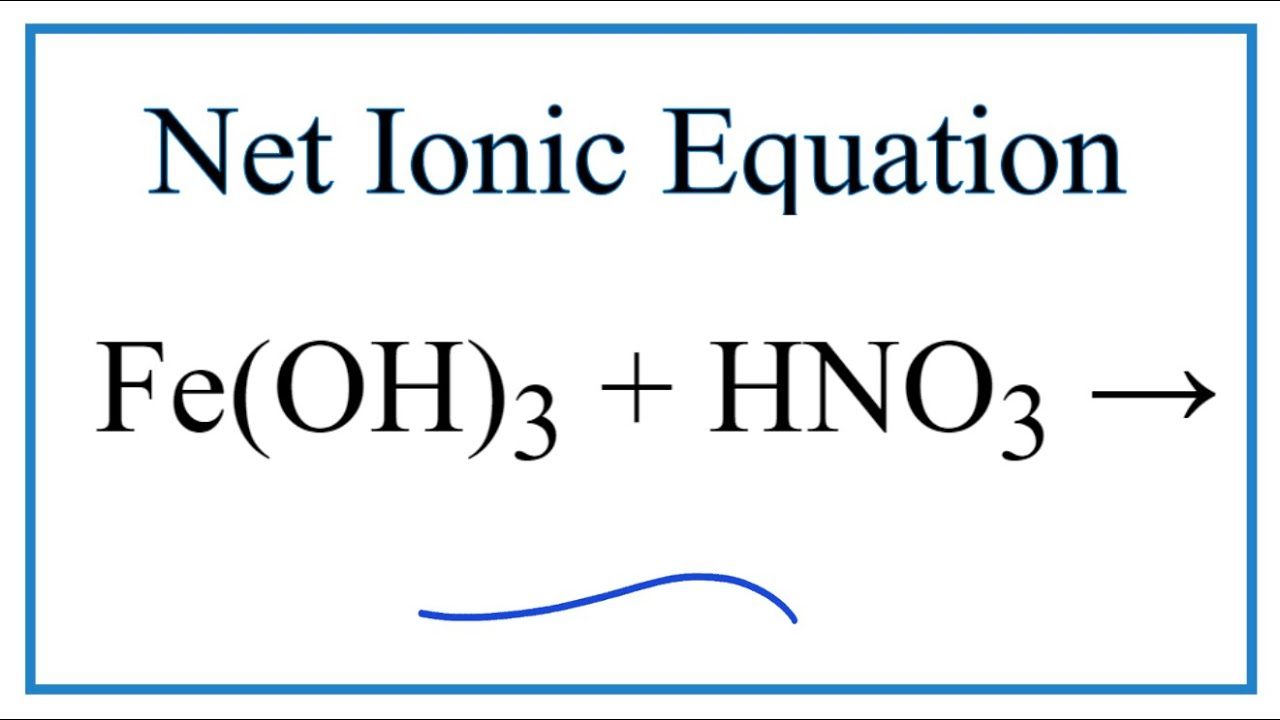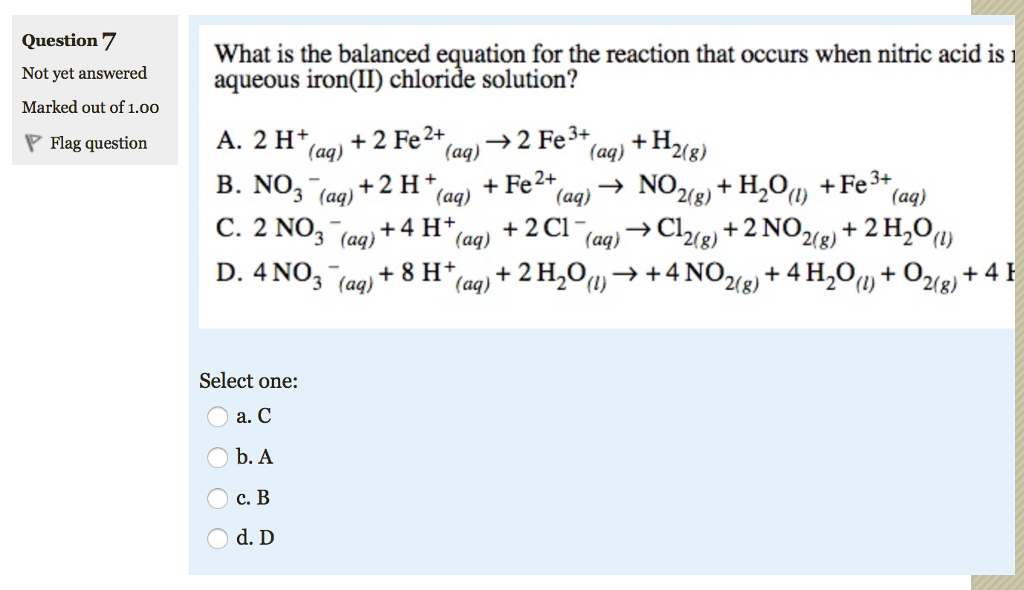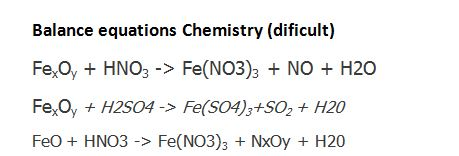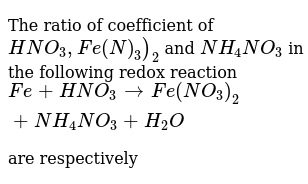
এর সহগের অনুপাতHNO(3), Fe(N)(3))(2)এবংNH(4)NO(3)নিম্নলিখিত redox প্রতিক্রিয়াFe + HNO(3) rarr Fe (NO(3))(2) + NH(4)NO(3) + H(2)O যথাক্রমে

I HNO3 + Fe= II HCl + Fe = please answer immediately don't send link - Science - Materials Metals and Non-Metals - 13482205 | Meritnation.com

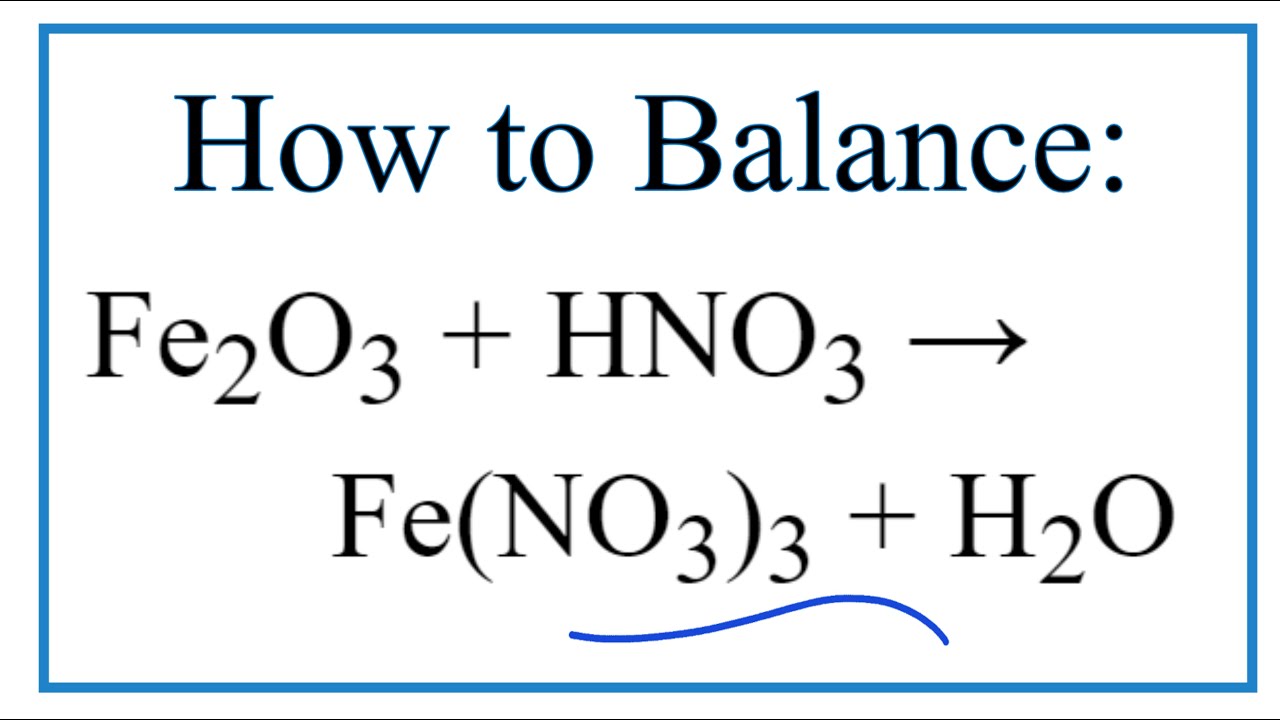
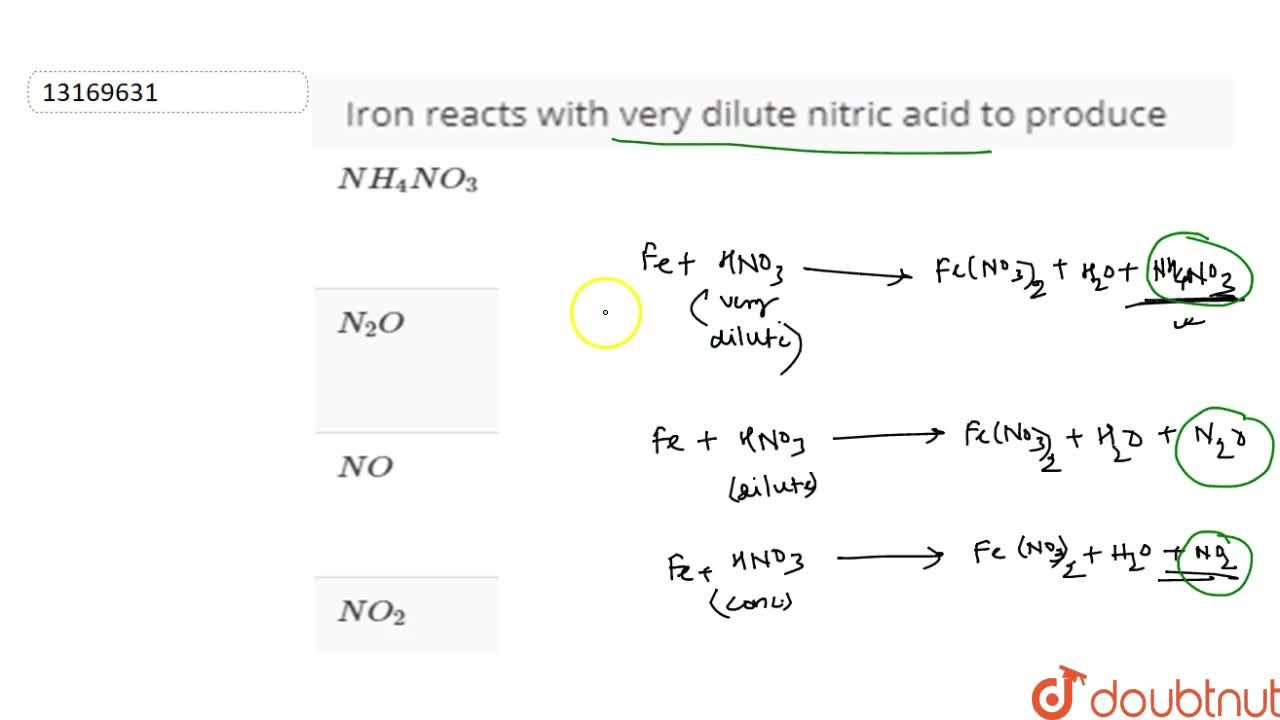
Solve the following equation by using ion electron method Fe(NO3)2 + HNO3 = Fe(NO3)3 +NO + H2O - Brainly.in

OneClass: 5. Balance the following equations, and name each reactant and product: (a) Fe2O3(s) + Mg(s...

Lakhmir Singh Chemistry Class 10 Solutions For Chapter 1 Chemical Reactions And Equations - Free PDF

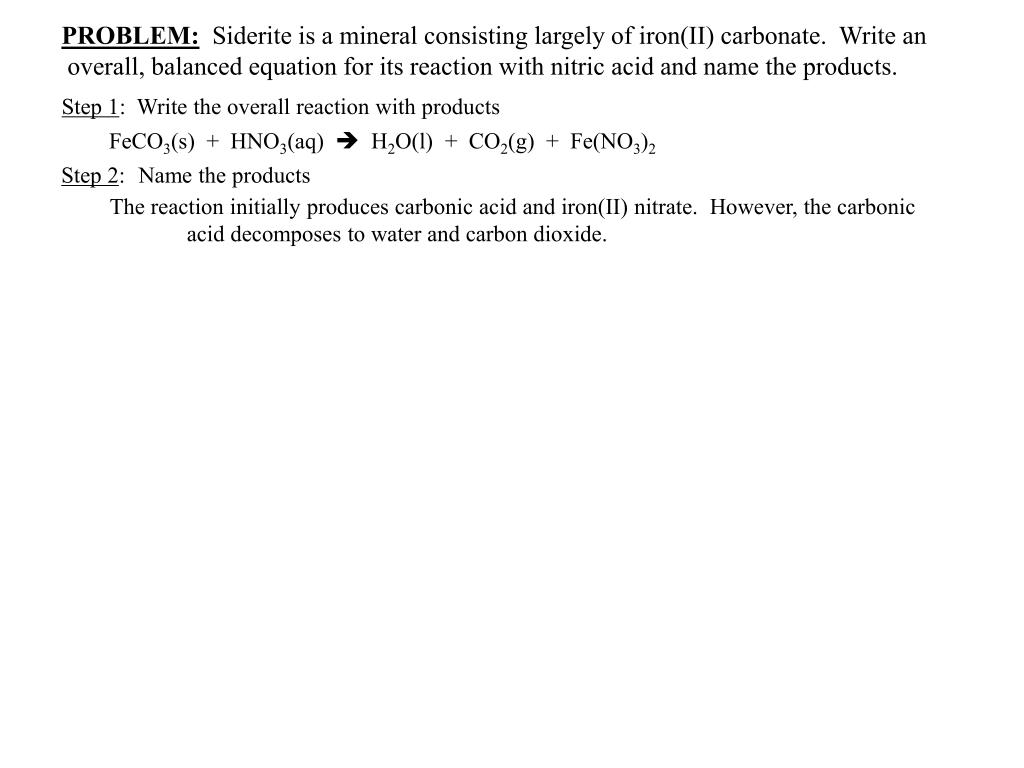
Balance the following equations a Fe H2O Fe3O4 H2 b Ca N2 Ca3N2 c Zn KOH K2ZnO2 H2 d Fe2O3 CO Fe CO2...

Balance the given equation by oxidation number method - FeSO4 + HNO3 + H2SO4 = Fe(SO4)3 + NO + - Chemistry - Redox Reactions - 13629296 | Meritnation.com