Can CAR-T and gene therapy cures really sustain biopharma? Not for all, analyst says | Fierce Pharma
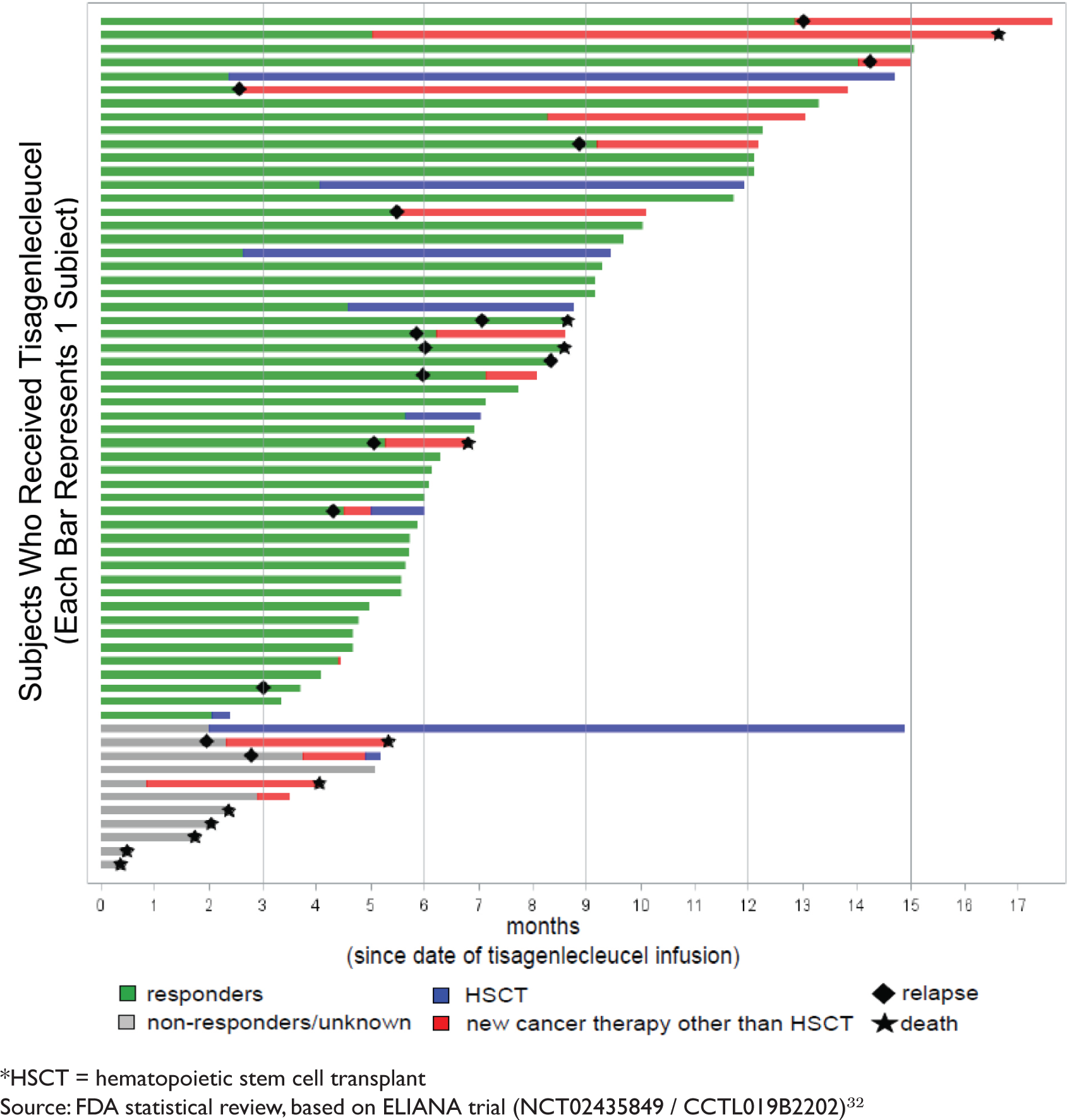
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
ONCOLOGIC DRUGS ADVISORY COMMITTEE BRIEFING DOCUMENT Tisagenlecleucel (CTL019) for the TREATMENT OF PEDIATRIC AND YOUNG ADULT PA

The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19 - Cytotherapy
ONCOLOGIC DRUGS ADVISORY COMMITTEE BRIEFING DOCUMENT Tisagenlecleucel (CTL019) for the TREATMENT OF PEDIATRIC AND YOUNG ADULT PA

Current state of U.S. Food and Drug Administration regulation for cellular and gene therapy products: potential cures on the horizon - Cytotherapy
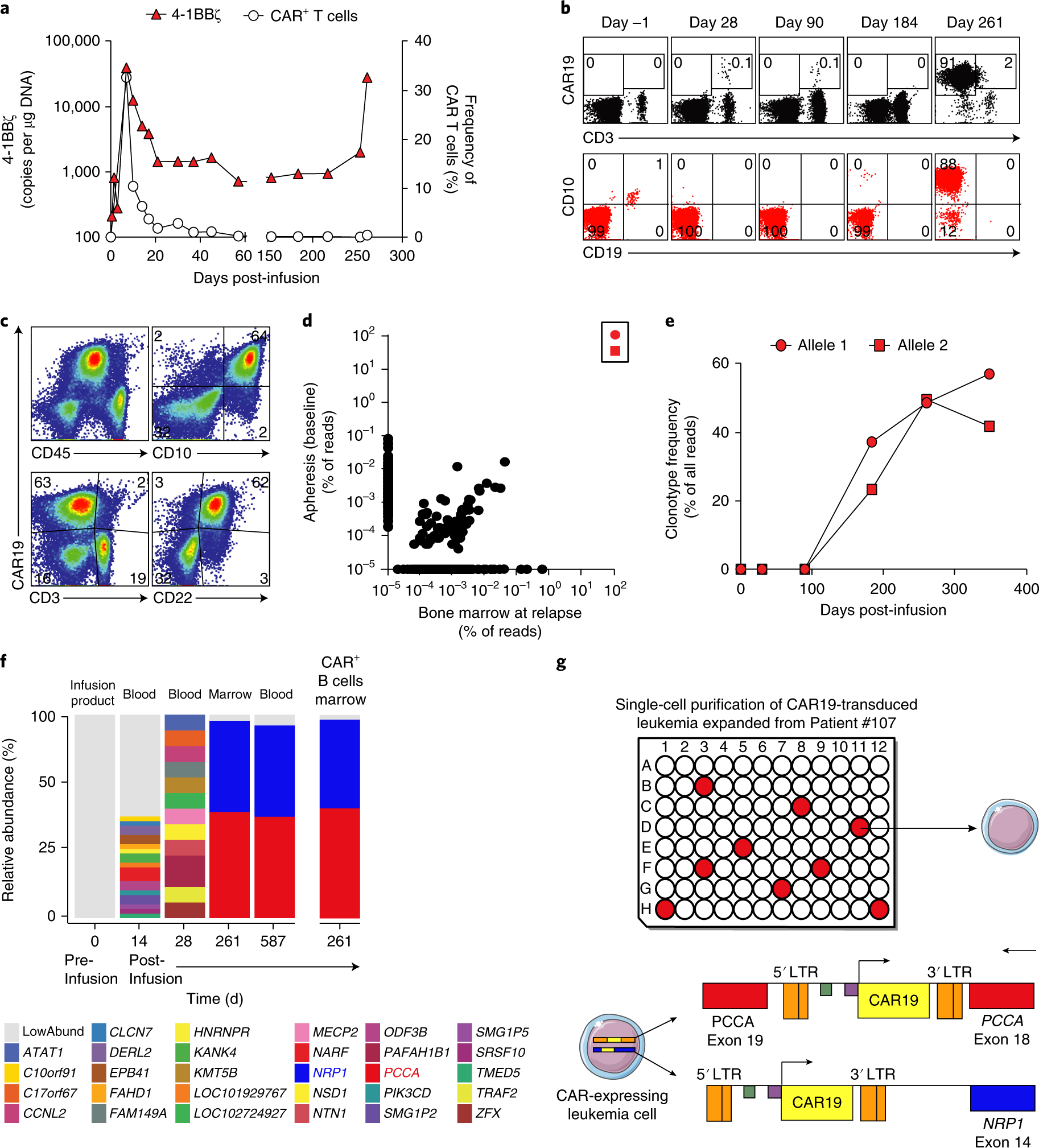
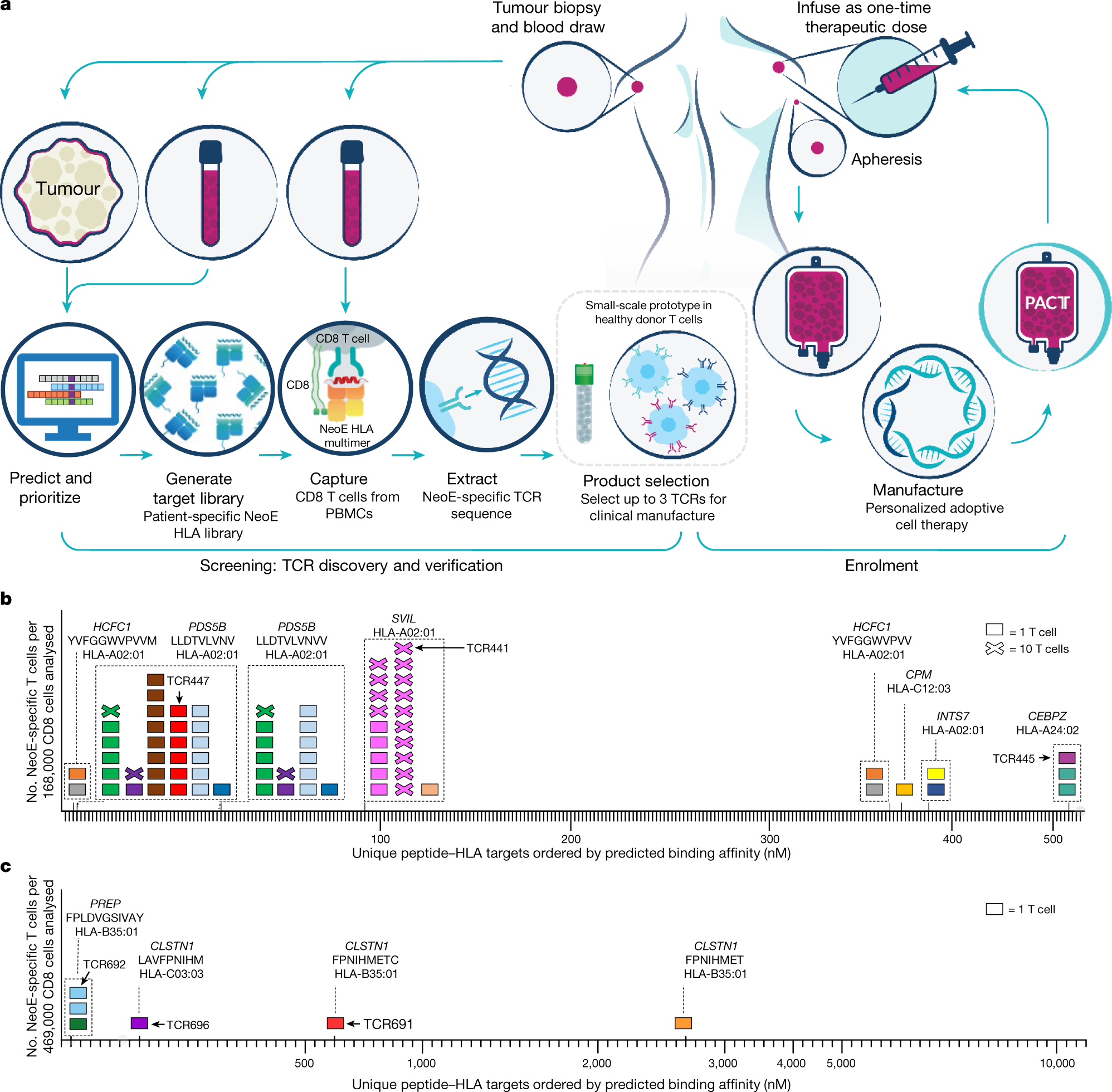
Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell | Nature Medicine
ASCO: Positive trial shows Novartis' Kymriah poised to play catch-up in CAR- T rivalry with Gilead's Yescarta | Fierce Pharma
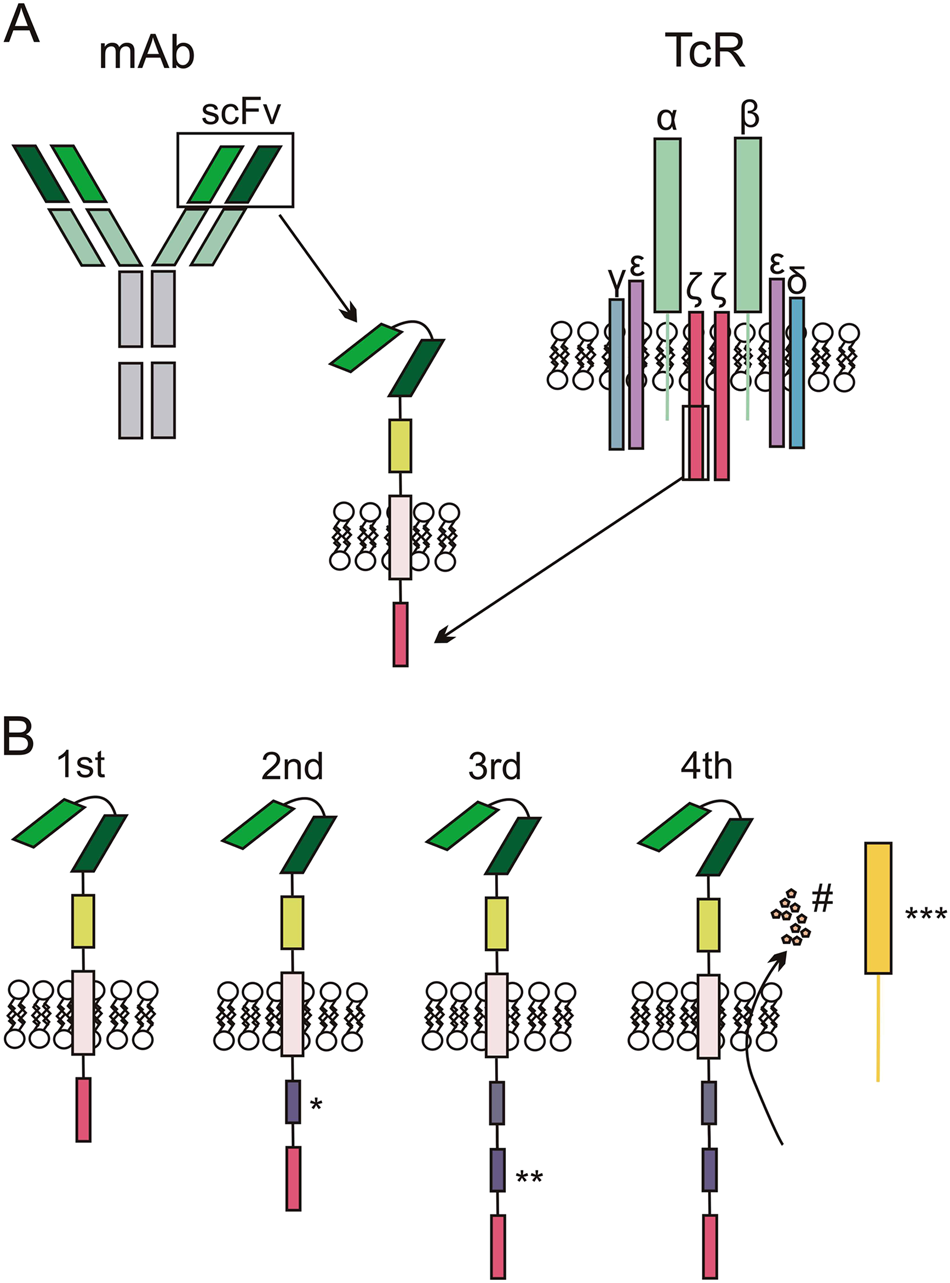
The biological basis and clinical symptoms of CAR-T therapy-associated toxicites | Cell Death & Disease
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development

Manufacturing News of Note—Novartis to have Cell Therapies make Kymriah in Australia; Piramal Pharma expands API plant in Canada | Fierce Pharma

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma | Nature Communications
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar