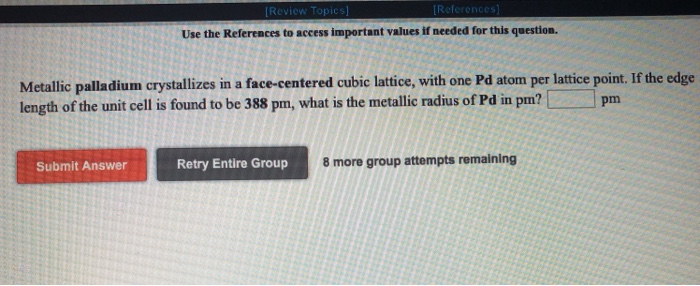
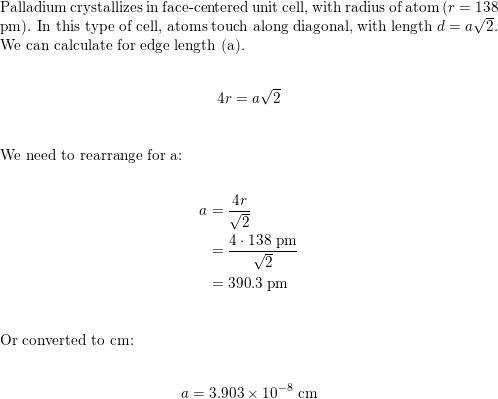SOLVED:Rhodium has a density of 12.41 g>cm3 and crystallizes with the face-centered cubic unit cell. Calculate the radius of a rhodium atom.
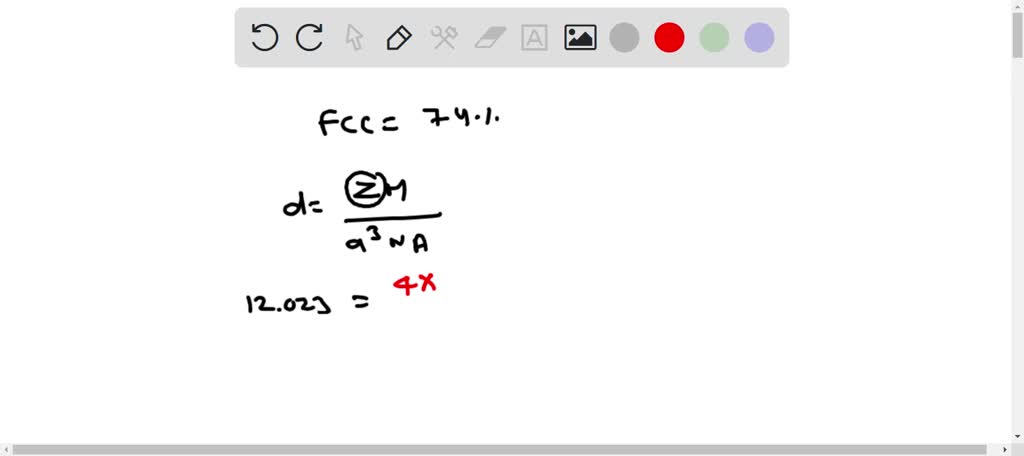
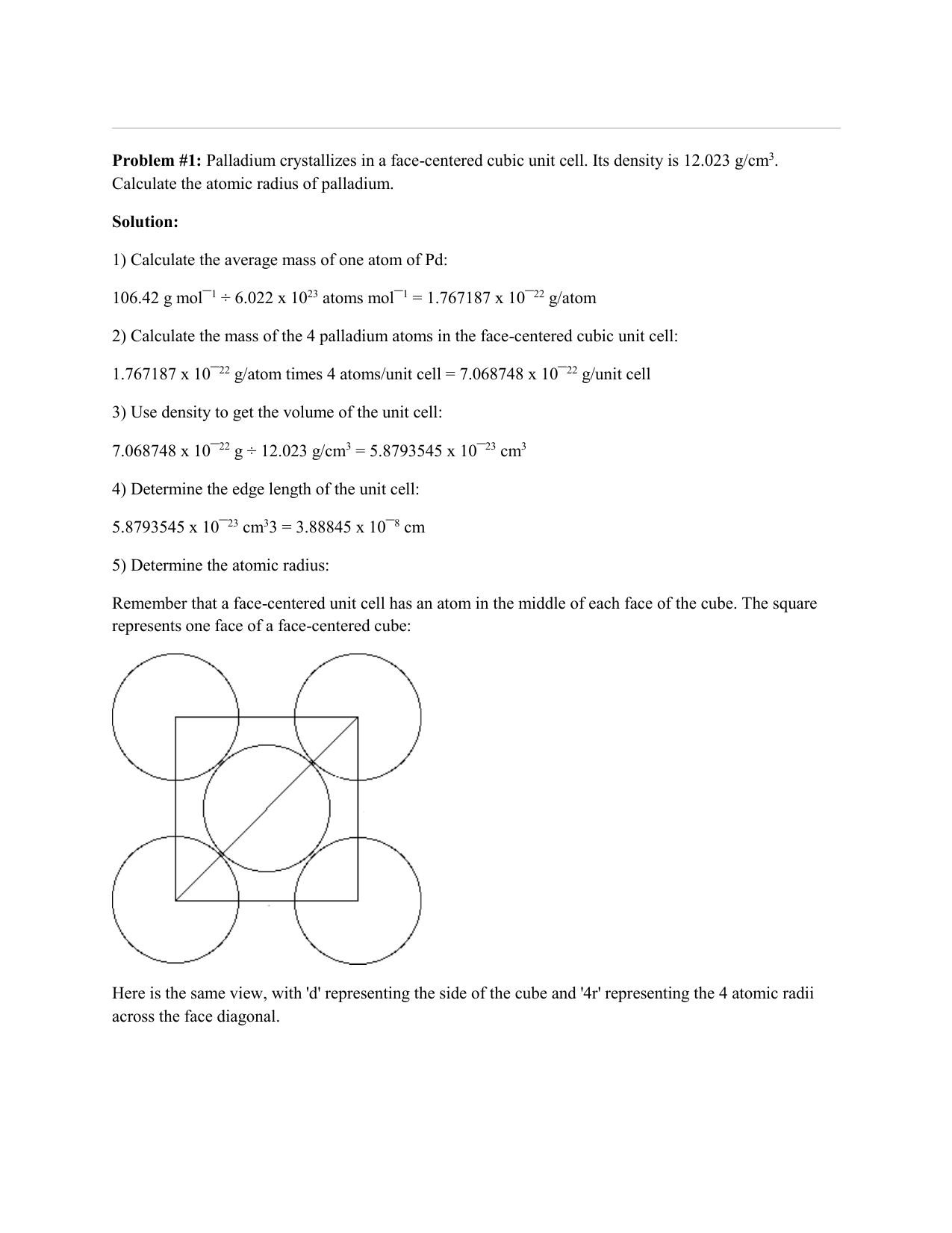
SOLVED: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius of palladium.

Copper has a face-centered cubic unit cell. How many atoms of Cu are present in each unit cell? | Homework.Study.com

OneClass: A metal crystallizes in the face-centered cubic (FCC) lattice. The density of the metal is ...

A metal crystallizes in the face-centered cubic unit cell with an edge length of 320 pm. \\ A. What is the radius of the metal atom? B. The density of the metal

An elemetnts crystallizes in a face centered cubic lattice and the edge of the unit cell i - YouTube
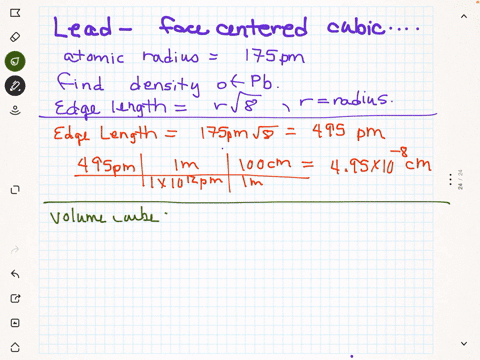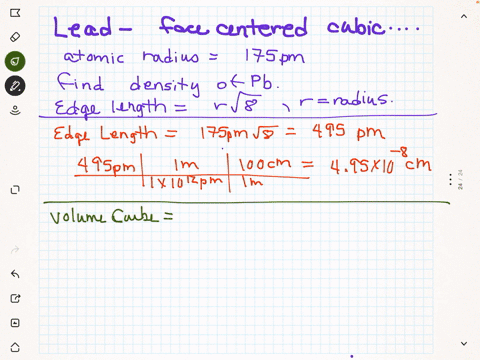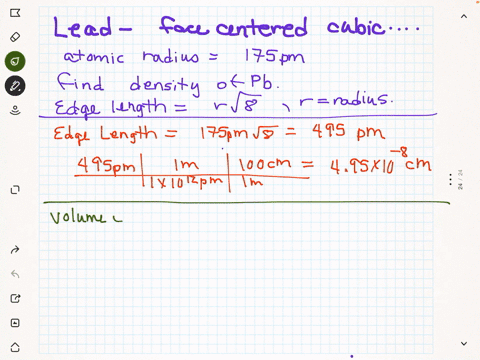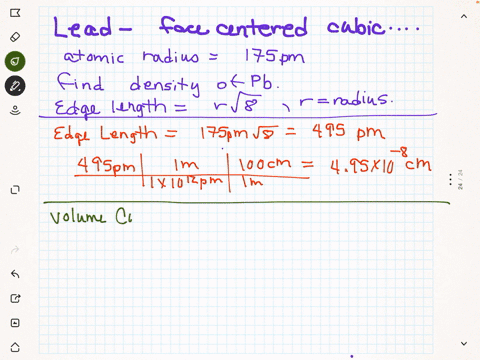
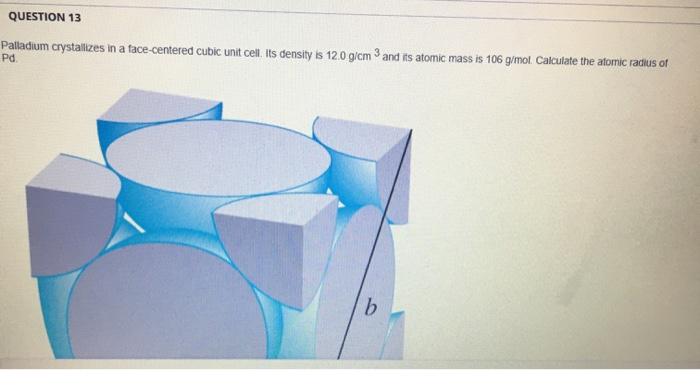
SOLVED: Palladium crystalllzes In face-centered cubic unit cell. Its density i5 12.0 g/cm 27"€. Calculate the atomic radius of palladium 138 pm (b) 1.95 * 10 " nm (c) 1.95 * 10 cm (d) 154 pmn (e) 0.109 nm

A metal crystallizes with a face -centred cubic lattice.The edge of the unit cells is `408 - YouTube

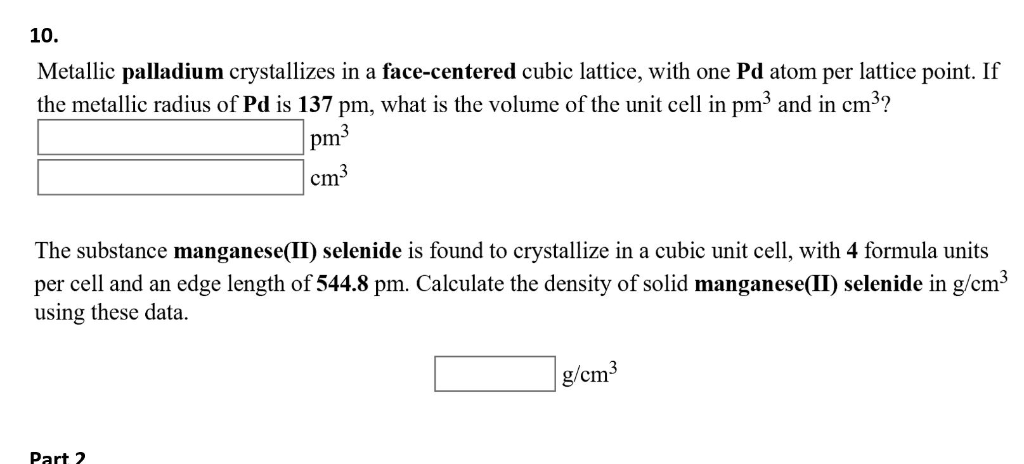
SOLVED: Metallic palladium crystallizes in a face-centered cubic lattice, with one Pd atom per lattice point. If the metallic radius of Pd is 137 pm, what is the volume of the unit

Document - Problem #1: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius of palladium. | Course Hero